Q:
Which compound matches the IR Spectrum?
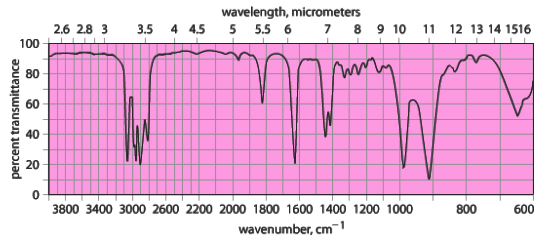
Answer & Explanation
Answer: A) 1, 5 – hexadiene
Explanation: 3 – Hexanol:
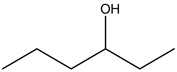
There is no alcohol stretch present in the spectrum. Therefore, the above structure is not suitable for the given spectrum.
Trans – 4 – octene:
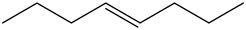
There is no vinyl bending present in the spectrum. Therefore, the above structure is not suitable for the given spectrum.
1, 5 – hexadiene:

The vinyl bending and other stretches were matched with the above structure.
Hence, the c = c stretch, vinyl bending and 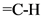 stretch were matched with the above structure.
stretch were matched with the above structure.
Therefore, the given spectrum belongs to 1, 5 – hexadiene.
View Answer
Report Error
Discuss